
CRISPR RNP-Based Genome Editing with cGMP Capacity
Aldevron now offers a full RNP* service solution to streamline your CRISPR reagents into therapies. Working with your unique guide RNAs (gRNAs), we’ve defined the optimal conditions for complexing, characterizing, and storing CRISPR RNPs created with Aldevron research and cGMP-grade CRISPR/Cas9 proteins.
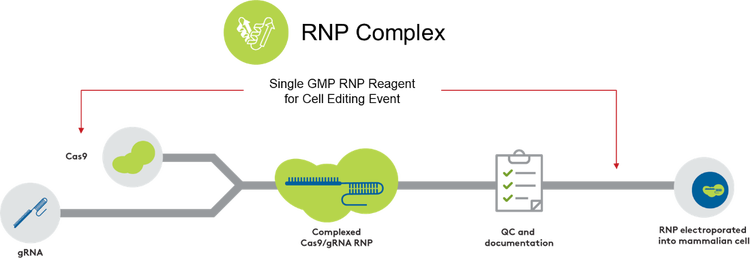
Aldevron provides multiple solutions for your CRISPR-associated gene editing program using RNPs. We have leveraged our expertise in CRISPR nuclease manufacturing to build a comprehensive RNP production service for your optimal final gene editing drug substance. This is done by utilizing either our readily available cGMP-grade nucleases or manufacturing a custom nuclease to your specific needs to produce the desired RNP complex.
- Explore our full list of Nuclease Products at Research and cGMP grade
- Aldevron offers Custom Manufacturing of unique Cas enzyme configurations including dCas9 fusions, nickases, Type-II variants and Type-V nucleases.
- Integrated DNA Technologies (IDT) RUO guide RNA and custom cGMP guide RNA are available through Aldevron, offering a seamless, single-point-of-contact solution for RNP complexes.
With our abundant offerings, Aldevron provides the highest quality and control for the scalable manufacturing of the nuclease and the combined RNP complex. We are dedicated to delivering reliability and lot-to-lot consistency for your RNP gene editing project by meeting industry standards and following regulatory guidance. Therefore, we aid in all stages of your workflow to complete IND submission through commercialization.
*Aldevron provides RNPs only to customers who are duly licensed, including to make and have made RNPs, for their intended use.
Learn more in our article about CRISPR RNPs and the Future of Cell and Gene Therapy
Regulatory Support
In therapeutic workflows, the composition of the CRISPR RNP complex needs to be well characterized. Aldevron uses novel, in-house methods to quantify activity and complexed RNP relative to uncomplexed Cas9 or gRNA. Our proprietary release panels are compliant with 21CFR210-211 and designed to meet current guidance from the FDA regarding gene editing components for human genome editing.
RNP QC test panel
| Product Characteristics | Concentration |
| Activity (in vitro) | |
| Appearance | |
| Residual free intact gRNA (Purity) | |
| Residual free Cas9 (Purity) | |
| pH | |
| Osmolality | |
| Critical Contaminants | Residual DNase |
| Endotoxin | |
| Bioburden or Sterility | |
| Mycoplasma |
- CASE STUDY: Enabling Novel CRISPR-Cas9 Delivery: A Case Study Supporting Innovative Genomics Insititute
- WHITEPAPER: Next-Generation CRISPR Approaches: Explore Strategies to Advance Therapeutic Programs Supporting Current Regulatory Guidance
- WHITEPAPER: Expectations on the Pathway to GMP for Gene-Modified Cell Therapies
- BROCHURE: Advancing CRISPR-based Therapeutic Development
When you start your research with us, you can travel one continuous path to finished product. We’ve designed and built facilities and capabilities to meet today’s needs, while continuing to build for tomorrow.

|

|

|
| Breakthrough Campus | Advance Campus | Aldevron Madison |
| Fargo, ND | Fargo, ND | Madison, WI |
|
Plasmid DNA, RNA, Protein manufacturing at cGMP & GMP-Source™ quality for clinical applications GMP Facility: 259,000 SF |
Plasmid DNA manufacturing for research applications; Process development Facility: 45,000 SF |
Protein manufacturing and process development for research and diagnostics Facility: 52,000 SF |
| Learn More | Learn More | Learn More |
Aldevron Advantage
During RNP development, Aldevron will:
- Work with our clients to identify the optimal complexing ratios, conditions, and formulation for their unique RNP.
- Provide our clients with complete manufacturing and analytical documentation in support of their IND filing process.
- Support our clients in their IND filing process regarding the manufacture of the RNP.