
pALD DNA Cloning Backbones
Find the Right Backbone for your Application
Aldevron, your trusted partner since 1998, has several cloning backbones available. These cloning backbones are empty vectors that can be modified to fit your needs. Each vector is available at research grade and is royalty and license free from research through commercial applications.
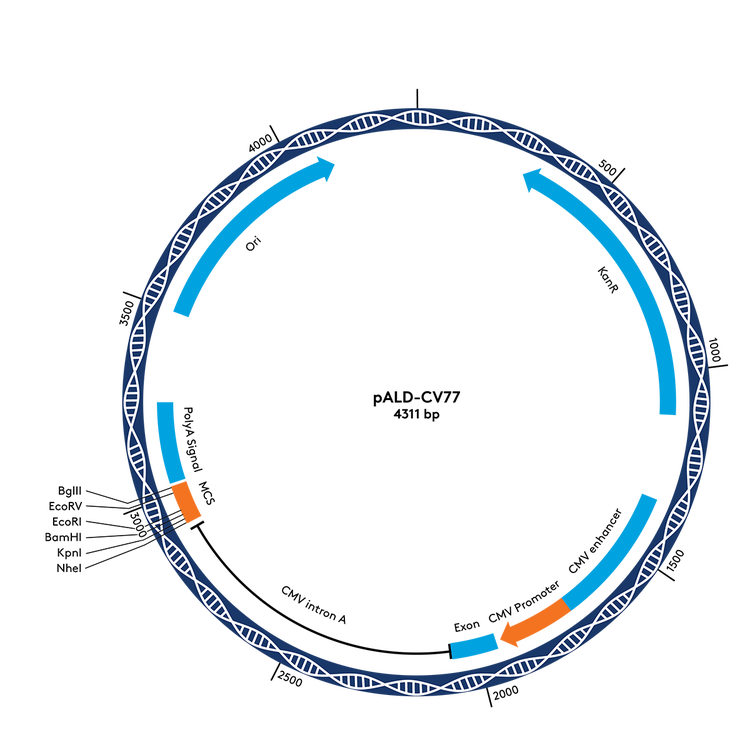
pALD-CV42 and pALD-CV77 are ideal for researchers who do not have a cloning backbone available. This construct set is appropriate for a wide range of applications. Researchers have also used the pALD-CV77 backbone sequence successfully in clinical trials.

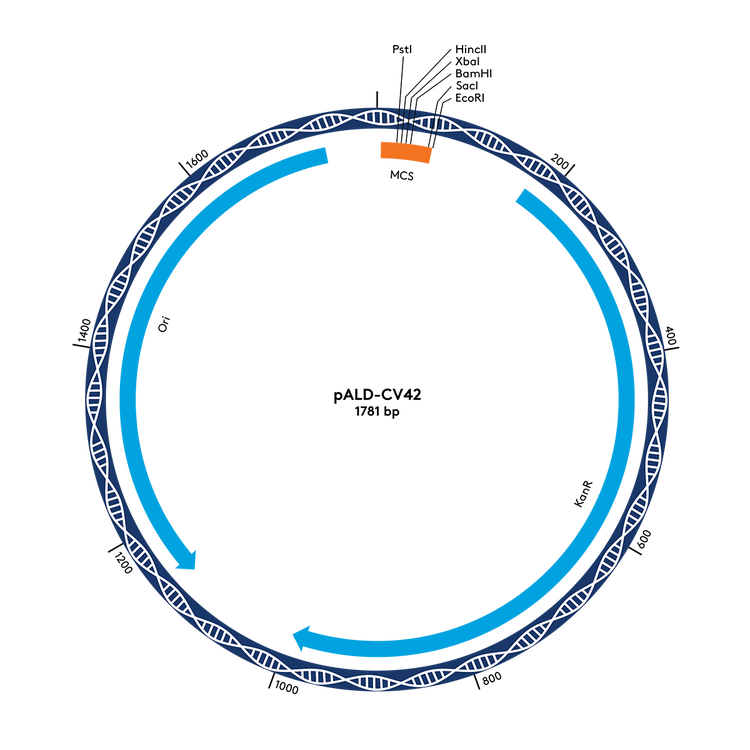
Aldevron now offers pALD-CV42 [T7] and pALD-CV42 [T7-PolyA]. These cloning backbones contain the T7 promoter region for RNA production, with an option for encoded poly(A) available in one construct.

![pALD-CV42 [T7] Map_WPRE](./media_1736ae03f7b03c35794b8f497b5ba7b5b9456ddc4.png?width=750&format=png&optimize=medium)
![pALD-CV42 [T7-PolyA] Map_WPRE](./media_1143ccdb0187ef3c18ce2201e6fe6a7ed91f78d08.png?width=750&format=png&optimize=medium)
Aldevron's cloning vectors are available as free samples. If you are interested, please fill out the Request Sample form.
Interested in rAAV Manufacturing? Check out rAAV specific ITR cloning vectors.
Ordering Information
| Appearance | Clear and Colorless |
| Buffer | TE |
| Concentration | 1.0mg/mL +/- 10% |
| DNA Homogeneity | Predominately Supercoiled |
| Endotoxin | ≤ 100 EU/mg |
| Identity | Confirmed Against DNA Ladder |
| Plasmid Identity | Identical to Reference Sequence |
| ABS 260/280 Ratio Purity | 1.80 – 2.00 |
| Residual Host Genomic DNA | ≤ 5% |
| Residual Host RNA | Not Visible at 200 ng Load |
| Restriction Digest | Matches Expected Restriction Pattern |
| Appearance | Clear and Colorless |
| Buffer | TE |
| Concentration | 1.0mg/mL +/- 10% |
| DNA Homogeneity | ≥ 80% Supercoiled |
| Endotoxin | ≤ 100 EU/mg |
| Identity | Size Confirmed Versus Supercoiled Marker |
| Plasmid Identity | Identical to Reference Sequence |
| ABS 260/280 Ratio Purity | 1.80 – 2.00 |
| Residual Host Genomic DNA | ≤ 5% |
| Residual Host RNA | Not Visible at 200 ng Load |
| Restriction Digest | Matches Expected Restriction Pattern |
Commonly Asked Questions
A: Yes. The pALD-CV77 cloning backbone is based on the pWRG7077 sequence which has been used in a number of clinical trials; please see references below (individual results may vary).
The pALD-CV42 cloning backbone is currently used in research applications.
NCBI:
A Phase 1 clinical trial of a DNA vaccine for Venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation
Examination of parameters affecting the elicitation of humoral immune responses by particle bombardment-mediated genetic immunization
Glycoprotein-Specific Antibodies Produced by DNA Vaccination Protect Guinea Pigs from Lethal Argentine and Venezuelan Hemorrhagic Fever
Clinicaltrials.gov:
