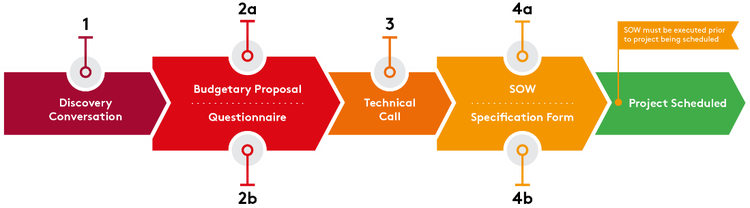
cGMP
There is no substitute for experience
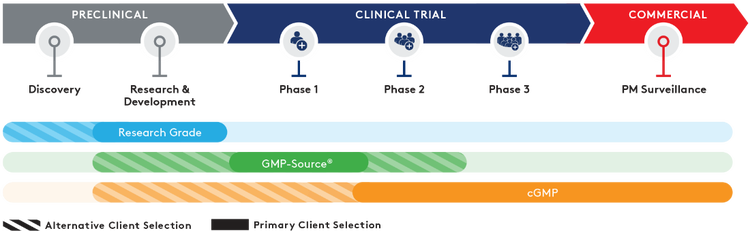
Congratulations! Your project is moving beyond early-phase trials. As you prepare to dose more patients, full cGMP manufacturing is required. When your materials are destined for clinical trial or commercial product, Aldevron’s cGMP services provide the highest quality oversight, process control, and can support any application.
Having supported many of our clients through clinical trials and regulatory filings. Aldevron has benefitted from years of health authority feedback, allowing us to navigate areas with limited guidance or regulation. That experience informs our clients’ regulatory strategies to help smooth the transition from pre-clinical through commercial applications.
Small-Scale cGMP manufacturing
Driven by a comprehensive quality assurance process, we produce cGMP products in segregated ISO-classified manufacturing suites, ensuring the highest quality products. Combined with dedicated project management and client-specific batch records, we work closely with our clients to provide customized cGMP plasmid products tailored to their unique requirements.
Our small-scale cGMP plasmid service is the right-sized process for clients who need ≤500mg for early-phase clinical trials. This service offers the flexibility to transition seamlessly into our traditional cGMP plasmid DNA manufacturing services for larger or pivotal trials, ensuring scalability and consistency throughout the development process.
We have also integrated our innovative Nanoplasmid™ vector technology into our small-scale cGMP Plasmid service. Nanoplasmids offer the distinct advantage of accommodating larger genetic payloads compared to traditional plasmid vectors, making them particularly well-suited for non-viral vector delivery methods. This advanced technology guarantees high yields, superior quality, and enhanced safety, providing a robust solution for complex therapeutic applications.
By leveraging the benefits of Nanoplasmid technology alongside our stringent cGMP standards, we provide you with the confidence and support required for successful therapeutic development and commercialization. Our comprehensive cGMP manufacturing capabilities are designed to offer versatile and scalable solutions that meet the evolving needs of cell and gene therapy applications.
Download the small-scale cGMP flyer
cGMP manufactured products offer:
- Screening for optimal growth conditions
- Growth via shake flask or high-density fermentation
- Alkaline lysis
- Chromatographic purification
- Consistent manufacturing process
- Certificate of Analysis (CoA)
- E. coli master cell bank generation
- Manufacturing Summary Report
- TSE/BSE statement
- Master Batch Records dictate process (controlled by Aldevron)
- ISO classified fill/finish with environmental monitoring
- QA oversight
- Development/engineering work prior to banking and growth
- Prescriptive change control process
- Project specific master batch records
- ISO classified production suites
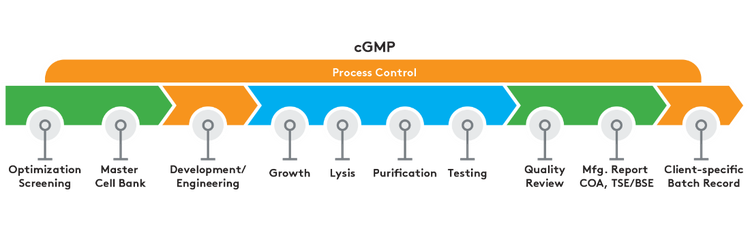
Our full cGMP fermentation capacity ranges from 10-liter to multiple 300-liter runs. This allows us to offer a complete spectrum of cGMP services, from early research to commercial supply.
Cell Bank
| Assay | Method |
| Host Cell Identity | Bacterial Colony Morphology |
| Host Cell Purity | TSA and SDA Plating |
| Cell Viability | CFU/mL plate count |
| Lytic Phage Contamination | Sample is co-inoculated with a qualified indicator strain on media containing no antibiotics |
| Plasmid Retention | Assessment of growth of isolated E. coli bacterial cell colonies on selective plate |
| Plasmid Copy Number | Digital PCR |
| pDNA Homogeneity | Agarose Gel Electrophoresis (AGE), T5-AGE, or Capillary Gel Electrophoresis (CGE) |
| Plasmid Identity | Sanger Sequencing |
Drug Substance
| Assay | Method |
| ABS 260/280 Ratio Purity | UV Spectrophotometry |
| Appearance | Visual inspection |
| Concentration | UV Spectrophotometry |
| DNA Homogeneity | Densitometry analysis of EtBr stained AGE |
| Endotoxin | Kinetic Chromogenic LAL |
| Identity | EtBr stained agarose gel electrophoresis |
| Plasmid Identity | Double Stranded Primer Walking Sequencing |
| Residual Host Genomic DNA | Quantitative PCR |
| Residual Host Protein | Micro BCA |
| Residual Host RNA | SYBR Gold stained agarose gel electrophoresis |
| Restriction Digest | EtBr stained agarose gel electrophoresis |
| Sterility | USP <71> Direct Inoculation |
*rProtein and mRNA are subject to further assay customization.
Ready to get started?
Start to finish, Aldevron team members show their commitment to client success through consistency of product, flexibility to meet your needs and schedules, and true cognitive empathy about the demands of the biotech world. As our industry expands at an almost exponential rate, we remain at your service.
To ensure we deliver the highest quality fundamental biologics you need, we have added an efficient and easy process for clients to enter our business lifecycle. By adding a robust front-end business process, we activate a quick-to-manufacture workflow, ensuring the project starts properly and ends successfully.